Researchers at the Case Western University School of Medicine say they have identified a previously unknown gene and associated protein which could potentially be suppressed to slow the advance of Alzheimer’s disease.
“Based on the data we have, this protein can be an unrecognized new risk factor for Alzheimer’s disease (AD),” said Xinglong Wang, an associate professor of pathology at the School of Medicine. “We also see this as a potential novel therapeutic target for this devastating disease.”
Wang said proving the latter assertion, which has not yet been tested in humans, would require additional research to corroborate the function of the protein they have dubbed “aggregatin.” Eventually, that would someday mean clinical trials with Alzheimer’s patients, he said.
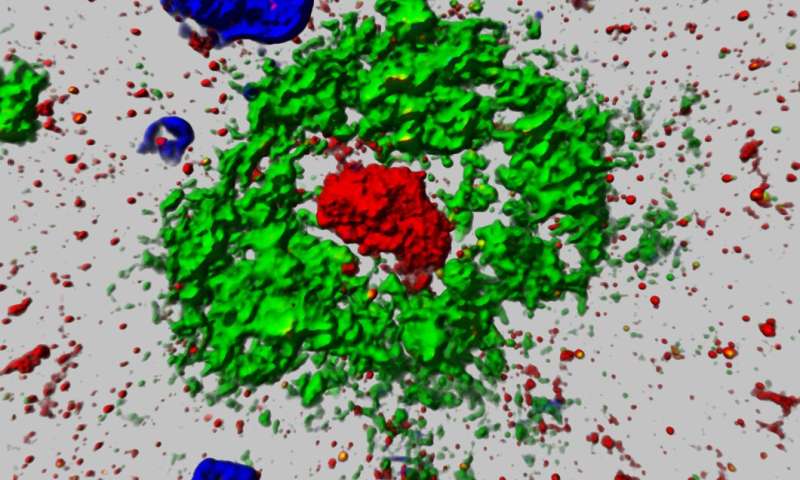
“This protein characteristically accumulates, or aggregates, within the center of plaque in AD patients, like the yolk of an egg—which is part of the reason we named it “aggregatin,” Wang said.
A research team led by Wang and Xiaofeng Zhu, a professor of Population and Quantitative Health Sciences at the School of Medicine, has filed for a patent through the university’s Office of Research and Technology Management for “novel Alzheimer’s disease treatments and diagnosis based on this and related study,” Wang said.
“We’re very excited about this because our study is likely the first systematic work combining the identification from a genome-wide association study of high dimensional brain-imaging data and experimental validation so perfectly in Alzheimer’s disease,” Zhu said.
Their research was published this month by the scientific journal Nature Communications and supported by grants from the National Institutes of Health (NIH) and the Alzheimer’s Association. Genomic and brain imaging data was obtained from the Alzheimer’s Disease Neuroimaging Initiative, which is supported by the NIH.
Alzheimer’s Disease affects millions
More than 5.7 million Americans have Alzheimer’s disease, which is the primary cause of dementia and sixth-leading cause of death in the United States. That population is predicted to reach 14 million by the year 2050, according to the Alzheimer’s Association.
The relationship between Alzheimer’s (and subsequent brain atrophy) and amyloid plaques—the hard accumulations of beta amyloid proteins that clump together between the nerve cells (neurons) in the brains of Alzheimer’s patients—has been well-established among researchers.
Less understood is precisely how that amyloid-beta actually leads to plaque formation—and where this new work appears to have broken new ground, Wang said.
Further, while there has been much research into what genes might influence whether or not someone gets Alzheimer’s, there is less understanding of genes that might be linked to the progression of the disease, meaning the formation of plaque and subsequent atrophy in the brain.
The role of ‘aggregatin’ protein
In the new work, the researchers began by correlating roughly a million genetic markers (called single-nucleotide polymorphisms, or SNPs) with brain images. They were able to identify a specific SNP in the FAM222, a gene linked to different patterns of regional brain atrophy.
Further experiments then suggested that the protein encoded by gene FAM222A is not only associated with AD patient-related beta-amyloid plaques and regional brain atrophy, but that “aggregatin” attaches to amyloid beta peptide—the major component of plaque and facilitates the plaque formation.
So when researchers injected mouse models with the “aggregatin” protein (made from the FAM222A gene), plaque (amyloid deposits) formation accelerated in the brain, resulting in more neuroinflammation and cognitive dysfunction. This happened, they report, because the protein was found to bind directly the amyloid beta peptide, thus facilitating the aggregation and placque formation, Wang said.
Conversely, when they suppressed the protein, the plaques were reduced and neuroinflammation and cognitive impairment alleviated.
Source: Read Full Article
