Based on scientific findings of the Netherlands Cancer Institute, a new combination treatment has been developed for patients with metastatic bowel cancer and a mutation in the BRAF gene. After a clinical trial in over 600 participants, those treated with this smart combination therapy survived longer than those who received standard treatment. The study is published today in the New England Journal of Medicine.
Every year, approximately 14,000 people in the Netherlands develop bowel cancer. Roughly one in 10 of them has a BRAF mutation. This means that they have a relatively poor prognosis, as their tumors respond very poorly to existing therapies once the cancer has spread. Unlike skin cancer patients with the same mutation, a medicine that inhibits BRAF does not work well in bowel cancer.
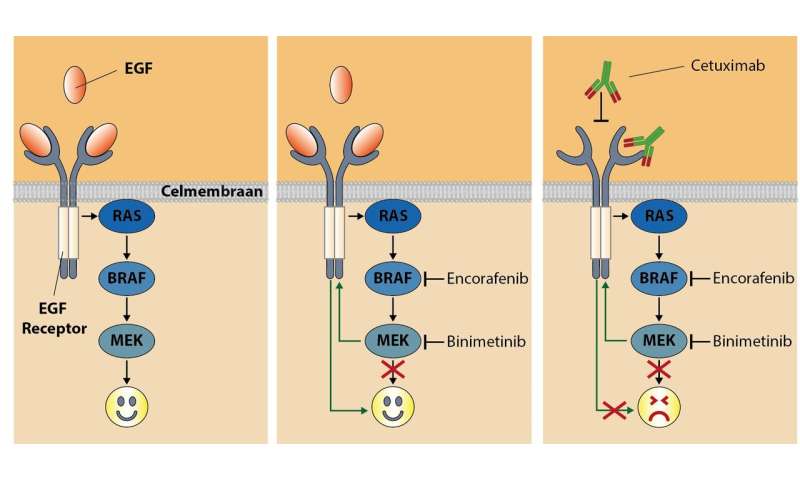
Blocking the detour
In 2012, researcher René Bernards and colleagues at the Netherlands Cancer Institute discovered why colon cancer cells are insensitive to BRAF inhibition. They showed that the growth signals in cells take a detour, which means that they can continue to function. This detour goes via the epidermal growth factor receptor EGFR (see illustration). Their recommendation was to give bowel cancer patients both a BRAF inhibitor and an EGFR inhibitor, in this way blocking the detour.
Following the first successful patient study at the Netherlands Cancer Institute, the BEACON study, a large international study, was set up for patients with metastatic bowel cancer and a BRAF mutation. The standard treatment for these patients is chemotherapy in combination with an EGFR inhibitor. The BEACON study compares this standard treatment with two experimental treatments that combine BRAF and EGFR inhibitors and exclude chemotherapy. Some of the patients also received a MEK inhibitor. The Netherlands Cancer Institute also participated in this study, under the supervision of Ph.D. student Sanne Huijberts, among others.
Extra time
The results show that patients who received the smart combination treatments survived longer: eight to nine months, compared with five months for standard treatment. In addition, in this group, it takes longer for the disease to start growing again: four months versus 1.5 months.
Oncologist Neeltje Steeghs from the Netherlands Cancer Institute says, “This is a unique combination of medicines for patients with a tumor that has not responded well to any drug at all until now. Ultimately, these tumors will also become insensitive to this combination treatment, but it does give patients a few extra months of time.”
At the end of last year, based on the promising intermediate results, the U.S. Food and Drug Administration gave the combination therapy a so-called “breakthrough therapy” designation. The FDA accelerates the registration such therapies as soon as the study is completed. The EMA is the equivalent body in Europe. Until the treatment is approved, it is unavailable.
Source: Read Full Article
