A drug used to treat and prevent HIV/AIDS is showing promise as a potential therapy for Alzheimer’s disease, and Vanderbilt University biochemist F. Peter Guengerich, Ph.D., is aiding efforts to make this approach to improving memory and cognitive function even better.
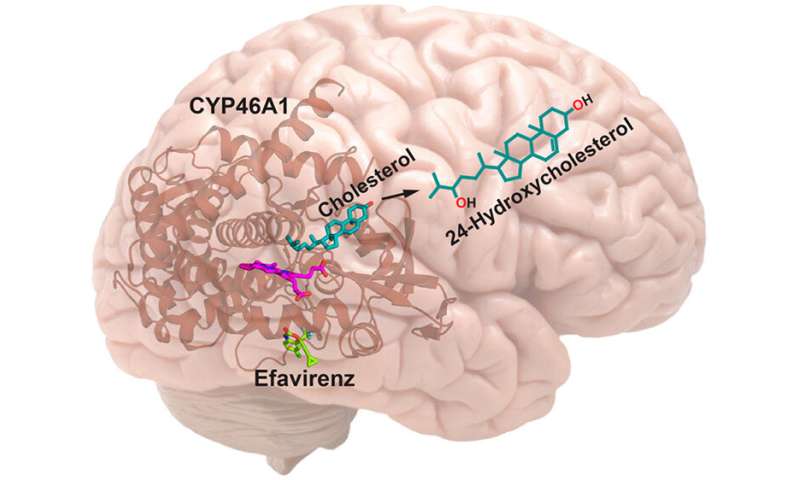
Last month in the Journal of Medicinal Chemistry, Guengerich and colleagues at Case Western Reserve University School of Medicine in Cleveland, Ohio, reported that structural modifications of the anti-AIDS drug efavirenz (EFV) can increase its ability to activate an enzyme which removes cholesterol from the brain.
Their paper was selected as an ACS “Editors’ Choice” by the scientific editors of journals published by the American Chemical Society, the world’s largest scientific society.
Cholesterol is thought to contribute to the formation of clusters or plaques of amyloid-beta protein that are the hallmark of Alzheimer’s disease. In the late 1990s the enzyme P450 46A1 (CYP46A1) was found to be important in breaking down and eliminating cholesterol from the brain.
More recently, Irina Pikuleva, Ph.D., professor of Pharmacology and Visual Sciences at Case Western Reserve, and colleagues reported that CYP46A1 could be activated pharmacologically in mice by low doses of EFV. They have begun a clinical trial of the drug in patients with mild cognitive impairment due to Alzheimer’s disease.
The therapeutic window for CYP46A1 activation by EFV in mice appears to be quite narrow, however. At higher doses, EFV inhibits the enzyme.
The goal of the current research is to generate a more potent compound that enhances the beneficial effects while avoiding potential side effects.
Guengerich, the Tadashi Inagami, Ph.D., Professor of Biochemistry, is one of the world’s most highly cited and productive researchers in biochemistry and pharmacology.
During the past 35-plus years, he and his colleagues have made major contributions to understanding how P450 enzymes metabolize drugs, carcinogens and other biological molecules including cholesterol.
Pikuleva, who completed her postdoctoral training at Vanderbilt, “has established a world-class research program at Case Western Reserve,” Guengerich said.
Five years ago, her team reported findings that suggest EFV activates CYP46A1 by binding to an allosteric site on the enzyme, a position other than the active site where cholesterol binds.
At higher doses, however, EFV is thought to bind both the allosteric and active sites. This inhibits the enzyme because it can no longer latch onto cholesterol.
Structure-function studies described in the current paper suggest that EFV metabolites, compounds which result from the breakdown of the drug in the body, may increase CYP46A1 activation without inhibiting the enzyme at higher concentrations.
Source: Read Full Article
