Heather Lieberman is betting that she will gain immunity from the coronavirus. Sandra Rodriguez wants to work with scientists for the good of the community.
Both have signed up for clinical trials of COVID-19 vaccines in Florida, the new US epicenter of the global health crisis.
“I want to be part of history. I want to help,” said Rodriguez, a 63-year-old teacher sitting in the offices of a clinical investigation center north of Miami, as nurses prepare to inject her.
“I want to do a good thing and I know this is a good thing. So I’m all for it.”
So-called phase three vaccine clinical trials—in which thousands of people take part in the final stages—are gaining traction in the Sunshine State.
With more than half a million cases and over 9,000 deaths, Florida ranks second in the United States in total cases behind California—making it an ideal place to carry out the trials.
That has led to a flurry of activity at the Research Centers of America (RCA), a private center carrying out clinical trials in Hollywood, 25 miles (40 kilometers) north of Miami,
RCA is working with six potential vaccines against COVID-19. Two of them, made by Moderna and Pfizer, are in phase three trials.
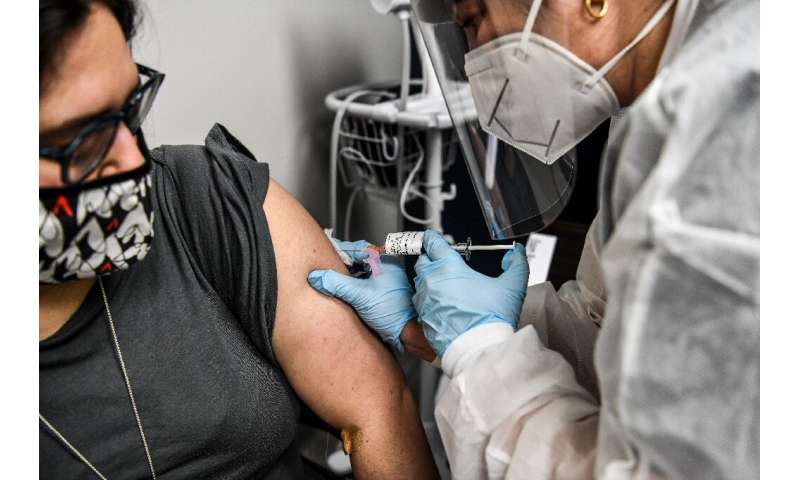
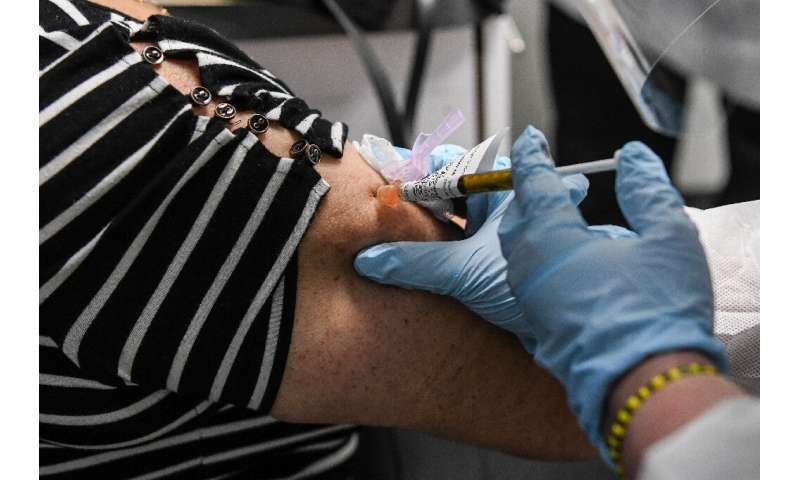
Volunteers come in one after the other, by appointment. They are examined by a doctor, sign a document and receive their injection.
They could be getting an experimental vaccine or just a placebo, because a control group is needed to establish a baseline for comparisons.
They are then asked to wait a couple of hours before being sent home once the experts have determined there is no adverse reaction.
“Our experience have been good. We haven’t had any issues in all the patients that we have vaccinated so far,” said Nelia Sanchez-Crespo, a doctor and researcher at RCA.
Volunteers have “been pouring in,” she told AFP.
“They’re really eager. I’ve seen much more desire to participate in these particular trials because people really want something to be available sooner.”
Seeking volunteers
That wish to see results, as well as the chance that they could gain immunity from the deadly disease, is what motivated the 28-year-old Lieberman.
“It’s worth trying,” she said as she waited at RCA.
“You can’t just live in a box,” she added, crossing her fingers that she does not end up in the control group.
Each day, RCA tests out a vaccine from a different lab.
On the day Lieberman and Rodriguez visited the clinic, they were testing mRNA-1273, developed by Moderna and the US National Institute of Allergies and Infectious Diseases (NIAID), which is led by high-profile government expert Anthony Fauci.
Moderna, a biotech firm set up less than a decade ago that has never brought a vaccine or drug to market, started its nationwide phase three trials on July 27.
That same day, US pharmaceutical giant Pfizer and its German partner BioNTech also announced that its vaccine BNT162b2 was advancing to phase two and three trials.
Each vaccine will be administered to 30,000 people in tests carried out in dozens of clinical research centers across the country.
Most of the tests will focus on California, Florida and Texas, the US states with the most cases.
Volunteers sign up on a web page and await a call from the clinic. Anyone is welcome.
“Each trial is looking for different things. We definitely want different groups,” said Sanchez-Crespo, who is hoping to receive hundreds of volunteers each week.
RCA is looking for “healthy individuals, patients that are first responders—doctors, nurses, firefighters—people who work in airports, restaurants. That’s very important. They have contact with a lot of people,” she said.
But she said that also needed are subjects in “high-risk groups because of their medical issues like diabetes, COPD, asthma.”
Sputnik
As well as the Moderna and Pfizer vaccines, a third contender, developed by Oxford University and AstraZeneca, has started phase three trials in the West.

Two more are at the same stage in China.
Russia surprised the world this past week when its leader Vladimir Putin announced that his country’s vaccine, named “Sputnik” after the pioneering 1950s Soviet satellite, offers “sustainable immunity” from the coronavirus.
Putin said that 20 countries had put in orders for a billion doses, but admitted clinical trials were not yet complete.
The news was met with skepticism by many Western scientists, who fear Russian researchers are cutting corners.
So far, the United States has poured more than $10 billion into six vaccine development projects and signed contracts to guarantee delivery of hundreds of millions of doses.
Source: Read Full Article
