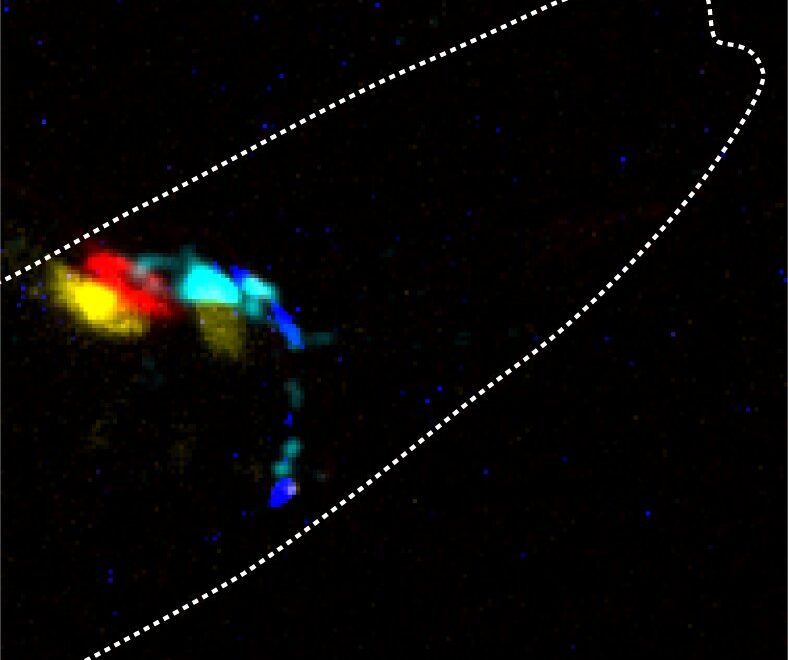
Our brains are made of billions of neurons, which are connected forming complex networks. They communicate between themselves by sending electrical signals, known as action potentials, and chemical signals, known as neurotransmitters, in a process called synaptic transmission.
Chemical neurotransmitters are released from one neuron, diffuse to the others and arrive at the targeted cells, generating a signal which excites, inhibits or modulates the cellular activity. The timing and strength of these signals are crucial for the brain to process and interpret sensory information, make decisions, and generate behavior.
Controlling the connections between the neurons would allow us to understand and treat better neurological disorders, rewire or repair the malfunctions of the neural circuits after being damaged, improve our learning capabilities or expand our set of behaviors.
There are several approaches to controlling neuronal activity. One possible method is using drugs, that alter the levels of the chemical neurotransmitters in the brain and affect the activity of neurons. Another approach is to use electrical stimulation applied to specific brain regions to activate or inhibit the neurons. A third possibility is using light to control neural activity.
Using photons to control the neuronal activity
Using light to manipulate neuronal activity is a relatively new technique that has been explored in the past. It involves genetically modifying neurons to express light-sensitive proteins, ion channels, pumps or specific enzymes in the target cells. This technique allows researchers to precisely control the activity of concrete populations of neurons with higher precision.
There are, however, some limitations. It needs to be delivered very close to the neurons to achieve enough resolution at the level of the synapsis, as light scatters in the brain tissue. Thus, it is often invasive, requiring external interventions. Moreover, the intensity needed to reach the targeted cells can be potentially harmful to them.
To overcome these challenges, a team of ICFO researchers presents in Nature Methods a system that uses photons instead of chemical neurotransmitters as a strategy to control neuronal activity.
The ICFO researchers Montserrat Porta, Adriana Carolina González, Neus Sanfeliu-Cerdán, Shadi Karimi, Nawaphat Malaiwong, Aleksandra Pidde, Luis Felipe Morales and Sara González-Bolívar led by Prof. Michael Krieg together with Pablo Fernández and Cedric Hurth, have developed a method to connect two neurons by using luciferases, light-emitting enzymes, and light-sensitive ion channels.
They have developed and tested a system named PhAST—short for Photons As Synaptic Transmitters—in the roundworm Caenorhabditis elegans, a model organism widely used to study specific biological processes. Resembling how the bioluminescent animals use photons to communicate, PhAST uses the enzymes luciferases to send photons, instead of chemicals, as transmitters between neurons.
Replacing chemical neurotransmitters with photons
To test if photons could codify and transmit the activity state between two neurons, the team genetically modified the roundworms to have faulty neurotransmitters, making them insensitive to mechanical stimuli. They aimed to overcome those defects using the PhAST system.
Secondly, they engineered light-emitting enzymes luciferases and selected ion channels that were sensitive to light. To follow the information flow, they developed a device that delivered mechanical stresses to the animal’s nose while measuring, at the same time, the calcium activity in the sensory neurons, one of the most important ions and intracellular messengers.
To be able to see the photons and study bioluminescence, the team had previously designed a new microscope by simplifying a fluorescence one, removing all the unnecessary optical elements such as filters, mirrors, or the laser itself, assisted with machine learning to reduce the noise coming from the external sources of light.
Researchers then tested that the PhAST system worked in several experiments and succeeded in using photons to transmit neuronal states. They were able to establish a new transmission between two unconnected cells, restoring neuronal communication in a defective circuit. They also suppressed the animals’ response to a painful stimulus, changed their response to an olfactory stimulus from attractive to aversive behavior and studied the calcium dynamics when laying the eggs.
These results demonstrate that photons can indeed act as neurotransmitters and allow communication between neurons and that the PhAST system allows the synthetic modification of animal behavior.
The potential of light as a messenger
Light as a messenger offers a broad scope for future potential applications. As photons can be used in other types of cells and several animal species, it has wide-ranging implications for both basic research and clinical applications in neuroscience.
Using light to control and monitor neuronal activity can help researchers better understand the underlying mechanisms of brain function and complex behaviors, and how different brain regions communicate with each other, providing new ways of imaging and mapping brain activity with higher spatial and temporal resolution. It could also help researchers develop new treatments, and for example, be useful for repairing damaged brain connections without invasive surgeries.
However, there are still some limitations to the widespread use of the technology, and further improvements in the engineering of the bioluminescent enzymes and the ion channels or in the targeting of molecules would allow controlling optically the neuronal function, non-invasively and with higher specificity and precision.
More information:
Michael Krieg, Neural engineering with photons as synaptic transmitters, Nature Methods (2023). DOI: 10.1038/s41592-023-01836-9. www.nature.com/articles/s41592-023-01836-9
Journal information:
Nature Methods
Source: Read Full Article