In a recent study published in the Nature journal, researchers assessed the impact of spatiotemporal epidural electrical stimulation (EES) on individuals suffering from chronic spinal cord damage.
The lumbar spinal cord contains the neurons that regulate the activity of walking. To activate these neurons responsible for walking, the brain transmits orders through descending pathways from the brainstem. An acute spinal cord injury (SCI) disperses this structured communication system. Although the injury does not cause direct harm to the lumbar spinal cord neurons, the lack of crucial supraspinal commands leaves them dysfunctional. This results in permanent paralysis. Individual case studies indicate that EES can rapidly reactivate these neurons present in the lumbar spinal cord, allowing paralyzed individuals to walk. Even when the stimulation was switched off, EES application during neurorehabilitation (EESREHAB) improved walking recovery.
 Study: The neurons that restore walking after paralysis. Image Credit: MattLphotography / Shutterstock
Study: The neurons that restore walking after paralysis. Image Credit: MattLphotography / Shutterstock
About the study
In the present study, researchers assessed the impact of EES on EESREHAB performed on individuals with chronic spinal cord damage.
First, the team examined whether EESREHAB could successfully restore walking in a broad population of SCI patients and whether this recovery required remodeling of the lumbar region. Next, the "Stimulation Movement Overground" (STIMO) trial determined the safety and viability of EESREHAB in improving walking recovery in chronic SCI patients. EESREHAB combined a surgically implanted neurostimulator with a multi-electrode paddle lead for closed-loop regulation of biomimetic EES protocols and overground neurorehabilitation assisted by a multidirectional robotic assistance system.
After the initial configuration phases, the participants completed EESREHAB for a total of five months, which included standing, walking, and executing various activities with the EES switched on (EESON) four to five times every week. Over time, participants' weight-bearing capacities increased, allowing them to walk outdoors with both EESON and a stabilizing device. Positron emission tomography (PET) of the uptake of 18F-fluorodeoxyglucose (FDG) was employed to measure the spinal cord's metabolic activity in response to walking before and after EESREHAB.
Based on the overexpression of immediate early genes, the team aimed to recognize neuronal subpopulations that were engaged using EESREHAB. The concept of prioritizing cell types was designed and verified. Furthermore, the machine learning method Augur was employed as a molecular compass to prioritize perturbation-responsive neurons in the lumbar spinal cord. This was conducted by first examining whether SCVsx2::Hoxa10 neurons were required to develop walking activity in healthy mice. To investigate the function of SCVsx2::Hoxa10 neurons following EESREHAB, the team introduced electrodes to the wireless system developed for spinal cord optogenetics. Redshifted micro-LEDs and electrodes were then incorporated in the same epidural implant to facilitate deeply penetrating photostimulation and allow walking using EES, respectively.
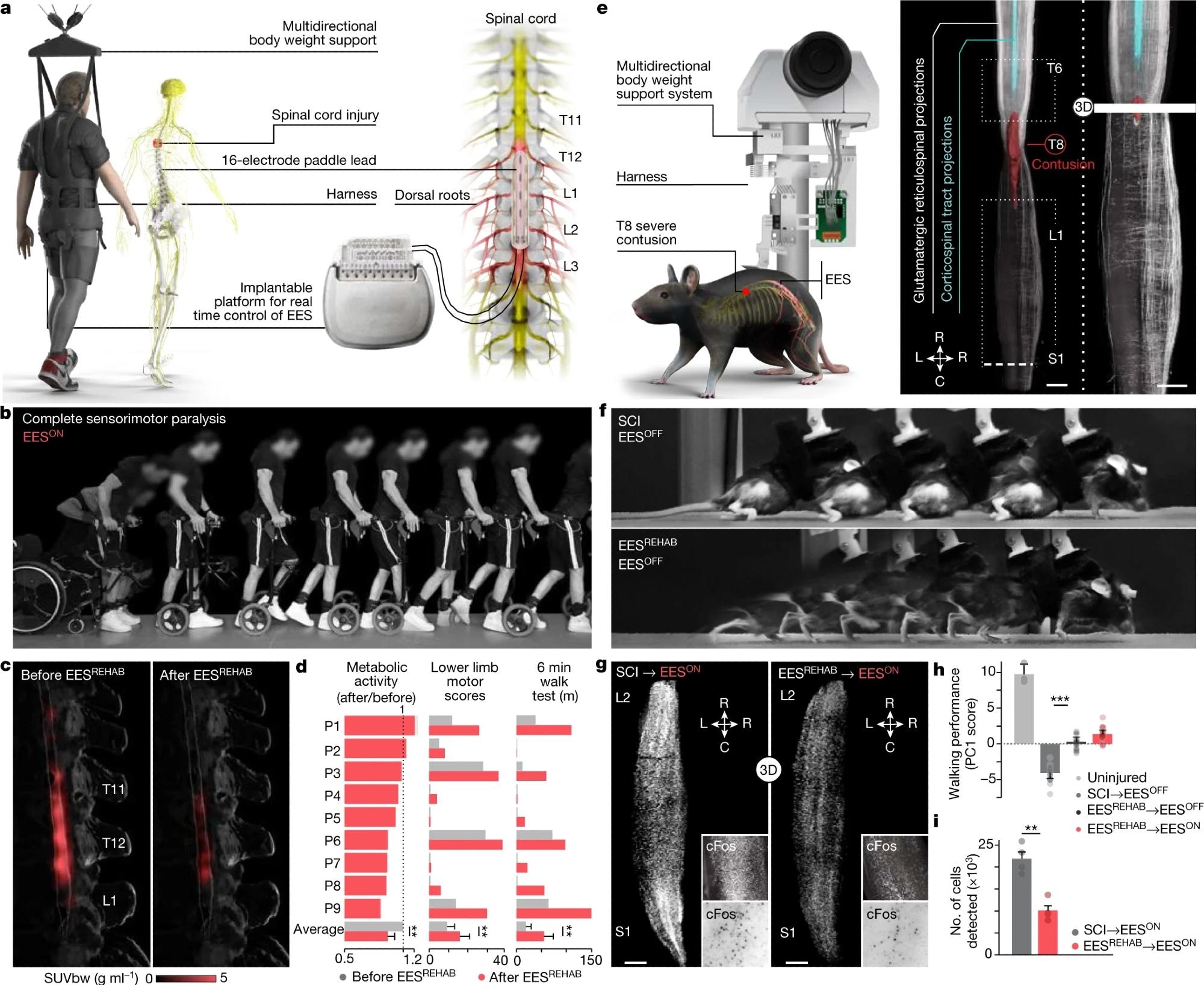 Body weight support system enabling overground walking and wireless implantable pulse generator operating in closed loop, connected to a paddle lead targeting the dorsal roots that innervate lumbosacral segments. b, Chronophotography showing the transitioning from sitting to walking in a representative participant. c, 18FDG-PET projected onto a personalized model of the spinal cord elaborated from high-resolution MRI (participant ID DM002), showing the metabolic activity of the spinal cord—expressed as standardized uptake value (SUVbw)—in response to walking before and after EESREHAB. d, Bar plots reporting the relative change in normalized FDG-PET metabolic activity during walking before and after EESREHAB, the lower limb motor scores, and the distance covered during the 6-min walk test (n = 9; metabolic activity mixed-effects model: t = −3.2, P = 0.002; lower limb motor scores, paired samples two-tailed t-test: t = 3.7, P = 0.0063; distance covered, paired samples two-tailed t-test: t = 3.5; P = 0.0076). e, Left, body weight support system enabling overground walking in mice, with implantable electrodes to deliver EES. Right, spinal cord visualization of projections from neurons in the motor cortex and glutamatergic (vGluT2ON) neurons in the reticular formation, traced with AAV5-CAG-COMET-GFP and AAV5-CAG-DIO-COMET-tdTomato, respectively. Scale bars, 1 mm. f, Chronophotography of representative mice with SCI only (SCI, EESOFF) or SCI with EESREHAB (EESREHAB, EESOFF). g, Lumbar spinal cord expression of cFos following walking with EESON following SCI or SCI with EESREHAB. Scale bars, 500 μm. h, Walking performance of uninjured mice (n = 3), mice with SCI (n = 10), and mice with SCI and EESREHAB tested with EESOFF (n = 10) or EESON (n = 10) (one-way ANOVA; Tukey’s honest significant difference for SCI versus EESREHAB→EESOFF: P = 3.3 × 10–11). i, The number of neurons expressing cFos(cFosON) (mice with SCI with EESON, n = 4; mice with EESREHAB and EESON, n = 4; independent samples two-tailed t-test: t = –5.7; P = 0.001). h,i, Bars show mean ± s.e.m. with individual points overlaid. *P < 0.05, **P < 0.01, ***P < 0.001.
Body weight support system enabling overground walking and wireless implantable pulse generator operating in closed loop, connected to a paddle lead targeting the dorsal roots that innervate lumbosacral segments. b, Chronophotography showing the transitioning from sitting to walking in a representative participant. c, 18FDG-PET projected onto a personalized model of the spinal cord elaborated from high-resolution MRI (participant ID DM002), showing the metabolic activity of the spinal cord—expressed as standardized uptake value (SUVbw)—in response to walking before and after EESREHAB. d, Bar plots reporting the relative change in normalized FDG-PET metabolic activity during walking before and after EESREHAB, the lower limb motor scores, and the distance covered during the 6-min walk test (n = 9; metabolic activity mixed-effects model: t = −3.2, P = 0.002; lower limb motor scores, paired samples two-tailed t-test: t = 3.7, P = 0.0063; distance covered, paired samples two-tailed t-test: t = 3.5; P = 0.0076). e, Left, body weight support system enabling overground walking in mice, with implantable electrodes to deliver EES. Right, spinal cord visualization of projections from neurons in the motor cortex and glutamatergic (vGluT2ON) neurons in the reticular formation, traced with AAV5-CAG-COMET-GFP and AAV5-CAG-DIO-COMET-tdTomato, respectively. Scale bars, 1 mm. f, Chronophotography of representative mice with SCI only (SCI, EESOFF) or SCI with EESREHAB (EESREHAB, EESOFF). g, Lumbar spinal cord expression of cFos following walking with EESON following SCI or SCI with EESREHAB. Scale bars, 500 μm. h, Walking performance of uninjured mice (n = 3), mice with SCI (n = 10), and mice with SCI and EESREHAB tested with EESOFF (n = 10) or EESON (n = 10) (one-way ANOVA; Tukey’s honest significant difference for SCI versus EESREHAB→EESOFF: P = 3.3 × 10–11). i, The number of neurons expressing cFos(cFosON) (mice with SCI with EESON, n = 4; mice with EESREHAB and EESON, n = 4; independent samples two-tailed t-test: t = –5.7; P = 0.001). h,i, Bars show mean ± s.e.m. with individual points overlaid. *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Lab Diagnostics & Automation eBook

Biomimetic EES methods enabled all nine subjects to restore or increase their walking capacity when supported by the robotic interface. Moreover, when EES was activated, the subjects, including two having total sensorimotor paralysis, could exert volitional control on the amplitude of their steps. Four subjects who had residual function prior to EESREHAB showed a significant improvement in their lower limb motor scores, restoring their ability to walk even without EES. These results supported the clinical trial's primary and secondary goals.
The improvement in walking function noted in the lower limb was reduced by EESREHAB within the lumbar segments. This decrease in neuronal activity within the lumbar spinal cord indicated that EESREHAB directed the activity-dependent selection of specific neuronal subpopulations required for walking following paralysis.
Two groups of excitatory lumbar spinal cord interneurons that expressed Vsx2 along with caudal spinal cord neurons (Hoxa10) (SCVsx2::Hoxa10) marker were selected in response to all of EESREHAB's therapeutic properties. This unanticipated discovery was independent of the resolution employed to characterize neuron subpopulations. The prioritized neurons were similar to developmentally characterized lumbar spinal cord V2a neurons. In response to walking using EES, the team detected an elevation in immediate early genes in SCVsx2::Hoxa10 neurons. This transcriptional activity was in contrast to the regulation of genes linked with long-term structural remodeling after EESREHAB.
When SCVsx2::Hoxa10 neurons were optogenetically inactivated in SCI-affected mice, there was an immediate inhibition of EES-induced walking. However, immediately after the micro-LEDs were switched off, walking resumed. In contrast, stimulation of SCVsx2::Hoxa10 neurons with chronic paralysis immediately phenocopied the fundamental aspects of the walking recovery observed in mice who had undergone EESREHAB, regardless of whether EES was activated. Chronic chemogenetic stimulation of SCVsx2::Hoxa10 neurons in mice undergoing rehabilitation without EES resulted in a walking recovery comparable to that of mice undergoing EESREHAB. It was hypothesized that persistent silencing of SCVsx2::Hoxa10 neurons would limit recovery in response to EESREHAB, since silencing these neurons inhibited walking after SCI.
Overall, the study findings demonstrated that EESREHAB restored walking and enhanced the neurological state of nine individuals with chronic SCI. This recovery illustrated the treatment effectiveness of EESREHAB in individuals with neurological deficiencies covering the full severity spectrum following SCI, thus paving the way for the clinical implementation of this therapy.
- Kathe, C., Skinnider, M.A., Hutson, T.H. et al., The neurons that restore walking after paralysis, Nature (2022), DOI: https://doi.org/10.1038/s41586-022-05385-7, https://www.nature.com/articles/s41586-022-05385-7
Posted in: Medical Science News | Medical Research News | Medical Condition News
Tags: Brain, Cell, Chronic, Clinical Trial, Cortex, Electrode, Epidural, Fluorodeoxyglucose, Genes, Machine Learning, micro, Neuron, Neurons, Optogenetics, Paralysis, Positron Emission Tomography, Spinal Cord Injury, Tomography, Walking

Written by
Bhavana Kunkalikar
Bhavana Kunkalikar is a medical writer based in Goa, India. Her academic background is in Pharmaceutical sciences and she holds a Bachelor's degree in Pharmacy. Her educational background allowed her to foster an interest in anatomical and physiological sciences. Her college project work based on ‘The manifestations and causes of sickle cell anemia’ formed the stepping stone to a life-long fascination with human pathophysiology.
Source: Read Full Article
