Through experiments on genetically modified mice, a team led by Ursula Quitterer, Professor of Molecular Pharmacology at ETH Zurich, determined which molecular switches are involved and how they need to be thrown to halt the malformations that damage the heart. One day, these insights could benefit people suffering from tetralogy of Fallot, provided substances are found that are capable of targeting and inhibiting BBLN or its interactions with other proteins. Quitterer and her team have already started searching for such substances.
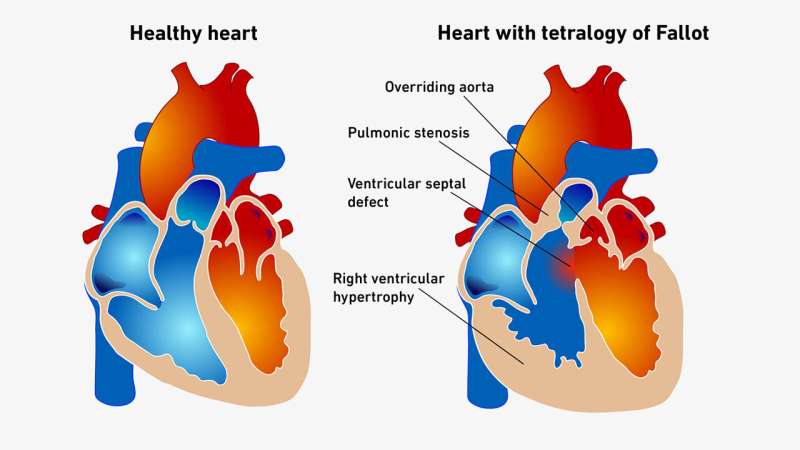
While tetralogy of Fallot is one of the most common congenital heart defects, this complex malformation is also exceedingly rare, affecting only 3 to 5 of every 10,000 newborn babies. For reasons that are still unclear, the affected individual’s heart has defects in a total of four places: a ventricular septal defect, which is a hole in the wall dividing the left and right ventricles; pulmonary stenosis, in which the blood flow to the pulmonary artery is obstructed; an overriding aorta with the major blood vessel deviating to the right; and right ventricular hypertrophy, in which the heart muscle on that side thickens over time.
Discoloration due to oxygen deficiency
Depending on their severity, these malformations can disrupt pulmonary circulation, causing the heart to pump blood returning from the rest of the body directly into the aorta instead of into the pulmonary artery. This means the blood cannot pick up oxygen in the lungs, which causes its normally red hemoglobin to turn a blue-violet color. “Babies suffering from acute tetralogy of Fallot are blue. You can actually see that they have oxygen deficiency,” says Quitterer.
Quitterer and her team have spent the past 15 years attempting to find out what the pathological mechanisms of tetralogy of Fallot are. Now, through experiments on genetically modified mice, they have managed to assemble some of the key pieces of the puzzle. This puts them in a position to create a more accurate picture of the complexity of a malformed heart. Their insights were recently featured in the journal Nature Cardiovascular Research.
Blank spot on the protein map
At the core of the investigations is a tiny protein, which Quitterer describes as “a blank spot on the protein map”—a protein that until recently didn’t even have a proper name. For the past two years, it has been known as the bublin coiled-coil protein (BBLN), a name it was given by Dutch researchers who discovered that nematode worms lacking the BBLN protein end up with tiny bubbles in their intestine.
Quitterer’s team encountered this little-researched protein in samples of heart tissue taken from toddlers who were born with tetralogy of Fallot and later operated on at a Cairo University hospital. Compared to tissue samples from young tetralogy of Fallot patients, which showed no discoloration, when the researchers examined heart tissue from “blue babies” they found a concentration of BBLN that was six times higher.
Morbidly enlarged rodent hearts
To discover what causes this upregulation, Quitterer’s team genetically modified some mice to produce human BBLN in their hearts. The higher the concentration of this protein, the more the hearts were enlarged—and the higher the incidence of heart failure. Further investigations shed light on the molecular interactions involved in orchestrating the right-sided thickening of the heart muscle.
“This unfortunate alteration, which makes a weak heart even weaker, also takes place in humans,” Quitterer explains. Today in wealthy countries like Switzerland, operations are often carried out on patients when they are still babies. And thanks to major advances in surgical technique, heart surgery can correct all four defects early on.
Such advances have already allowed medicine to vastly improve the life expectancy of people with this condition. But the pathological mechanisms in question continue to affect heart cells even after an operation. “As a result, tetralogy of Fallot patients who have had their hearts repaired still have a higher risk of long-term complications such as heart failure,” Quitterer says.
More information:
Joshua Abd Alla et al, BBLN triggers CAMK2D pathology in mice under cardiac pressure overload and potentially in unrepaired hearts with tetralogy of Fallot, Nature Cardiovascular Research (2023). DOI: 10.1038/s44161-023-00351-6
Journal information:
Nature Cardiovascular Research
Source: Read Full Article
