Immunotherapy is a type of cancer treatment that boosts the immune system to fight cancer by helping it to recognize and attack tumor cells. While this therapeutic strategy continues to revolutionize the treatment of an increasing number of tumor types, it fails to achieve clinical responses in many patients and does not yield benefit in the treatment of many cancers. There is therefore an unmet clinical need to identify predictive biomarkers of response for selecting those patients who would most likely benefit from immune-based therapies.
Published in the journal Med, results of a study led by researchers of VHIO’s Cancer Genomics Group led by Ana Vivancos, and our Research Unit for Molecular Therapy of Cancer (UITM)—CaixaResearch directed by Elena Garralda, have preliminarily validated the VIGex gene expression signature as a tool for classifying solid tumors based on the expression level of genes involved in the adaptive immune response, and a predictor of patients’ response to immunotherapy.
“Over the past decade, the development of immune-based strategies to treat cancer patients has led to profound anti-tumor activity across several tumor types. Various PD-1/PDL-1 checkpoint inhibitors have subsequently been approved for multiple different cancers. However, there is an unmet need for robust predictive biomarkers that can identity patients most likely to benefit from these therapies,” says first author Alberto Hernando-Calvo, a Clinical Investigator of VHIO’s UITM-Caixa Research and Medical Oncologist at the Vall d’Hebron University Hospital’s (HUVH) Medical Oncology Department.
“To address this limitation, we developed a pan-cancer platform to measure the activation of genes implicated in the antitumor immune response. We analyzed the gene expression of more than 1000 samples from VHIO’s institutional molecular prescreening program, our Advanced Molecular Diagnostics Program supported by the FERO Foundation,” explains Ana Vivancos, Principal Investigator of VHIO’s Cancer Genomics Group and corresponding author of this present study.
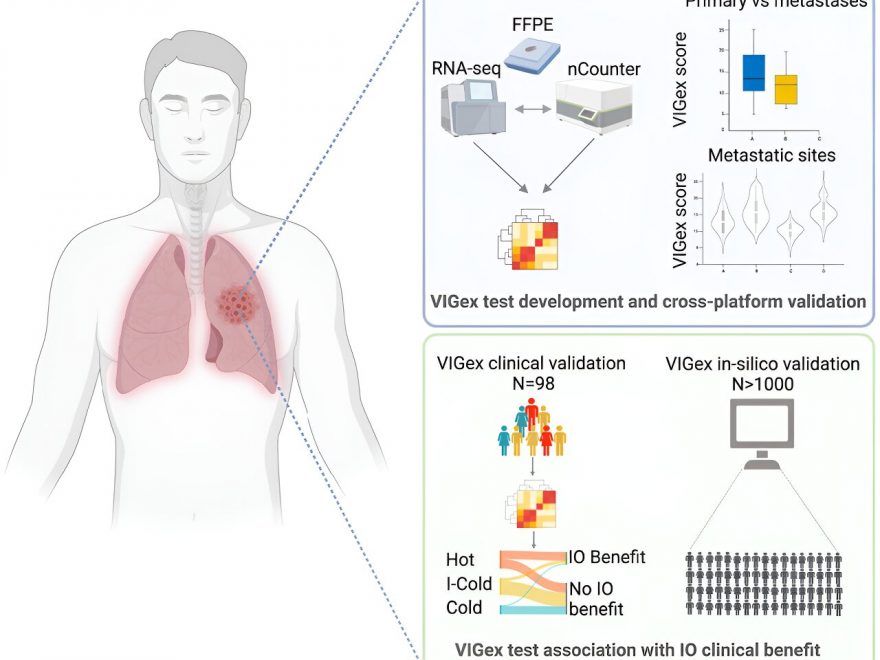
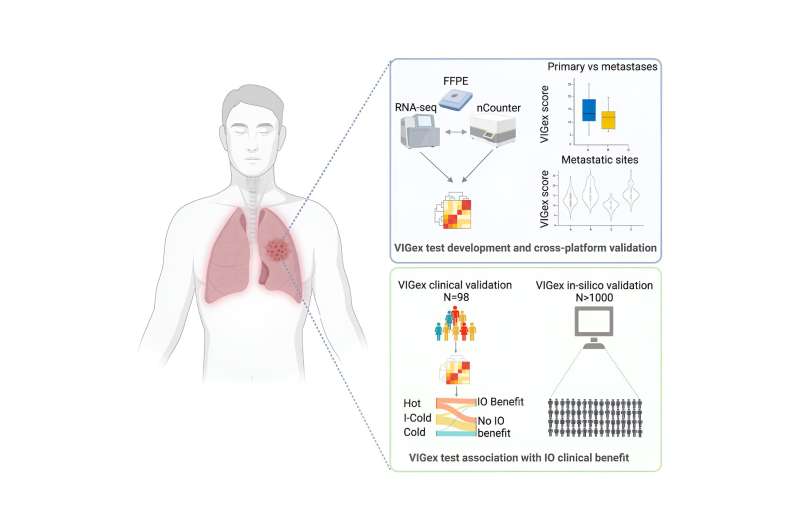
VIGex, developed in-house as a predictive platform to help guide patient selection for immunotherapy, is an optimized gene signature based on the expression level of 12 key genes implicated in the activation of the immune response.
This novel tool has been designed to classify solid tumors into three categories based on the inflammatory status of the tumor microenvironment: hot, intermediate-cold and cold. PD-1/PD-L1 checkpoint inhibitors (ICIs) have been shown to work best against hot tumors, while they generally fail to yield benefit in the treatment of cold tumors.
The inflammatory status of the tumor microenvironment, scored by the VIGex signature, varied depending on the origin of the primary tumor and anatomic site of metastases, with liver metastases showing an immunosuppressive tumor microenvironment. This last observation justifies the worse immunotherapy outcomes observed in patients with liver metastases across histologies.
The predictive power of VIGex was initially observed in a cohort of 98 refractory solid tumors from patients treated in early phase immunotherapy clinical trials performed at VHIO’s UITM-CaixaResearch. The investigators subsequently validated the test in a metanalysis with more than 800 tumor samples from previously published studies with clinical and gene expression datasets available to the scientific community.
In the initial validation cohort of patients treated with immunotherapy agents under development in early phase clinical trials, the authors observed better responses in those patients with tumors classified as hot.
“In addition to measuring the inflammatory status of the tumor microenvironment, we generated a pan-cancer biomarker platform that integrated VIGex categories with the gene expression levels of immunotherapy targets in early phase drug development,” adds Vivancos.
According to Elena Garralda, Director of VHIO’s UITM-CaixaResearch and a co-author of this present study, “the development of this tool will improve the selection of patients who are more likely to benefit from immuno-oncology across tumor types and different immune-based treatments. As part of this international collaboration, our VIGex signature will be applied to data from an immunotherapy clinical trial conducted at the Princess Margaret Cancer Centre in Toronto.”
VIGex will also be used as a biomarker in a prospective clinical trial of the European Organisation for Research and Treatment of Cancer (EORTC), for head and neck cancer patients treated with immunotherapy.
“Our results support the clinical utility of VIGex as a tool to aid oncologists in patient selection for trials testing personalized immunotherapy. In the future, this could lead to the expansion of VIGex as a predictive marker for novel immuno-oncology combinations in early phase clinical studies,” concludes Ana Vivancos.
More information:
Alberto Hernando-Calvo et al, A pan-cancer clinical platform to predict immunotherapy outcomes and prioritize immuno-oncology combinations in early-phase trials, Med (2023). DOI: 10.1016/j.medj.2023.07.006
Journal information:
Med
Source: Read Full Article