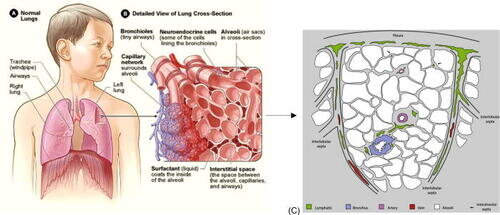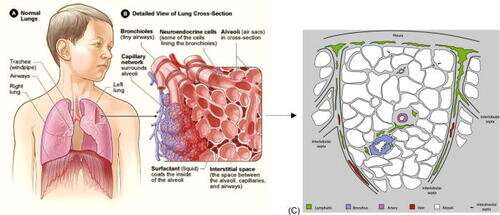
Pulmonary edema, a buildup of fluid in the lungs that can be fatal, presents a 125-year-old medical puzzle—one that has now been solved by researchers at the University of Michigan (U-M) and Arts et Métiers ParisTech. Their work is published in APL Bioengineering.
The finding could shed light on the workings of the lung at a time when respiratory infections such as COVID-19 and RSV are filling hospitals.
As the lungs perform their task of taking in oxygen and expelling carbon dioxide, fluid can accumulate in air sacs of certain patients. The fluid can make it harder for the lungs to perform that gas exchange, and at worst it can bring oxygenation levels low enough to cause death.
But for patients who recover from pulmonary edema, we haven’t really known how the fluid leaves the lungs. It has been established that the fluid drains to distant parts of the lymph system. What isn’t known is how it gets there.
In 1896, medical doctor and lung anatomist Dr. William Snow Miller discovered that lung air sacs do not have nearby lymphatics. The nearest lymph vessels were more than a tenth of a millimeter away—an epic journey compared to lymphatic system access in much of the rest of the body. And for more than a century, neither Miller nor anyone else has been able to determine how or why fluid from the air sacs can travel that far.
Enter James Grotberg, a U-M professor of biomedical engineering, and Francesco Romano, an associate professor of fluid mechanics at Arts et Métiers ParisTech and a former postdoctoral researcher in Grotberg’s lab.
What drew you to take a look at this long-standing mystery?
Grotberg: As the pandemic was bringing respiratory disease into global focus, I thought we might find important insights into the pulmonary edema that continues to cause COVID-19 deaths. My expertise spans the intersection of fluid dynamics and engineering as well as human biology and emergency and critical care medicine, so I am well positioned to explore this problem. Meanwhile, Francesco excels at computational fluid dynamic models, so we teamed up to develop a model for pulmonary edema with support from the National Institutes of Health.
How did you go about solving this?
Grotberg: Since no one had done this before, we started with fundamental flow physics and physiology. The fluid is serum leaking from the blood vessels into the air sacs—that’s pulmonary edema. Meanwhile, the lymphatic system acts like a vacuum, drawing excess fluid out of the tissues. But the lymphatics were far away, too far for their suction to be effective. Given that problem setup, we used mathematical modeling to simulate both how pulmonary edema builds up and how the body clears it.
What did you find?
Grotberg: Our model revealed a mechanism that can drive excess fluid from the air sacs to these distant lymphatics. It’s a new physiological flow. We discovered that fluid pressures in the tiny region between air sacs and capillaries is quite different from medical physiology textbook assumptions, and those pressure differences can drive fluid toward the lymphatic system.
How do you think this information can be useful going forward for treating pulmonary edema?
Grotberg: Any set of concepts underlying diagnosis and therapy has to include all the relevant phenomena. The new flow we discovered is a key ingredient. We believe it’s relevant to both major causes of pulmonary edema: elevated lung blood pressure, a symptom of congestive heart failure, and the breakdown of the air-blood barrier as can occur with infections like COVID-19 and RSV. In both scenarios, excess fluid in the air sacs blocks normal oxygenation and can be lethal.
We hope that our model can provide insights on how to treat pulmonary edema. It takes as inputs the anatomical and physiological parameter values of the lung, as well as clinical interventions, and then simulates how the entire system works.
For instance, an essential component of therapy may be treating the infection with antimicrobial agents—but also keeping the fluid balance well tuned to remove the excess fluid and restore normal oxygen delivery. Our model can predict how the fluid levels in the lungs will respond to changing conditions.
Source: Read Full Article