NOTICE: This Consumer Medicine Information (CMI) is intended for persons living in Australia.
STELARA (201116) ACMI
This medicine is subject to additional monitoring due to approval of an extensionof indications. This will allow quick identification of new safety information. Youcan help by reporting any side affects you may get. You can report side effects toyour doctor, or directly at www.tga.gov.au/reporting-problems.
Ustekinumab (rmc)
Consumer Medicine Information
What is in this leaflet
This leaflet answers some common questions about STELARA (pronounced stel-ahr-uh). It does not contain all the available information. It does not take the place of talking to your doctor, nurse or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using STELARA against the benefits this medicine is expected to have for you.
If you have any concerns about using STELARA, ask your doctor or pharmacist.
It is important that you read this leaflet.
Keep this leaflet with your medicine. You may need to read it again.
What STELARA is used for
STELARA is a prescription medicine that is used to treat:
adults and paediatric patients (children and adolescents) 6 years and older with moderate to severe plaque psoriasis that is chronic (doesn’t go away),
adults with active psoriatic arthritis, an inflammatory disease of the joints that is usually accompanied by psoriasis,
adults with moderately to severely active Crohn’s disease, an inflammatory disease of the bowel.
adults with moderate to severe ulcerative colitis, an inflammatory disease of the bowel.
STELARA contains the active ingredient ustekinumab, a monoclonal antibody. Monoclonal antibodies are proteins that recognise and bind to other unique proteins.
Ustekinumab blocks the action of two proteins in your body called interleukin 12 (IL-12) and interleukin 23 (IL-23). IL-12 and IL-23 are made by your body’s immune system. In people with psoriasis, psoriatic arthritis, Crohn’s disease or ulcerative colitis, IL-12 and IL-23 can cause their immune system to attack normal healthy parts of their body. Ustekinumab can block IL-12 and IL-23 from causing the immune system to attack.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is available only with a doctor’s prescription.
Before you use STELARA
When you must not use it
Do not use STELARA if you have an allergy to:
ustekinumab, the active ingredient in the medicine
any of the other ingredients in STELARA. See Product Description at the end of this leaflet for a list of ingredients.
Symptoms of an allergic reaction may include rash, itching or hives on the skin, shortness of breath, wheezing or difficulty breathing, swelling of the face, lips, tongue or other parts of the body.
Do not use STELARA if the packaging is torn or shows signs of tampering, or if the liquid is discoloured or cloudy.
Do not use STELARA beyond the expiry date (month and year) printed on the pack.
Do not use STELARA if you know or think that it may have been exposed to extreme temperatures (such as being accidentally frozen or heated).
Before you start to use it
You must tell your doctor about all of your medical conditions before each treatment, including if you:
have any kind of infection, even if it is very minor
have an infection that won’t go away or a history of infection that keeps coming back
have had TB (tuberculosis), or if you have recently been near anyone who might have TB
have or have had any type of cancer
have any new or changing lesions within psoriasis areas or on normal skin
have recently received or are scheduled to receive a vaccine. Patients receiving STELARA should not receive live vaccines. Tell your doctor if anyone in your house needs a vaccine. The viruses in some vaccines can spread to people with a weakened immune system, and can cause serious problems
are receiving allergy immunotherapy.
are pregnant, planning to become pregnant, or breastfeeding. Adequate contraception should be used to avoid falling pregnant. STELARA should only be used during a pregnancy if needed. Women who are breastfeeding should talk to their doctor about whether or not to use STELARA
have an allergy to latex.
Your doctor will examine you for tuberculosis (TB). If your doctor feels that you are at risk for TB, you may be treated with medicine for TB before you begin treatment with STELARA and during treatment with STELARA.
If you have not told your doctor or pharmacist about any of the above, tell them before you start using or are given STELARA.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including medicines you can buy without a prescription from a pharmacy, supermarket or health food shop.
Using STELARA
For the treatment of psoriasis or psoriatic arthritis STELARA is given by injection just under the skin (subcutaneously).
For the treatment of Crohn’s Disease and ulcerative colitis, the first dose of STELARA will be given by an intravenous infusion, which means that the medicine will be given to you through a needle placed in a vein. After this starting dose, all future doses of STELARA are given by injection just under the skin (subcutaneously).
All STELARA injections are for single use in one patient only.
STELARA is intended for use under the guidance and supervision of your doctor. In children and adolescents 6 years and older with psoriasis, it is recommended that STELARA be administered by a health care provider. When you start treatment, STELARA may be injected by your healthcare provider. If your doctor determines that it is appropriate, you or your caregiver may be able to administer it under the skin yourself if you wish, after proper training in injection technique. (See How much to use and Injecting STELARA under the skin yourself).
It is important to remember that the first dose of STELARA for the treatment of Crohn’s Disease or ulcerative colitis (intravenous infusion) will always be administered by your healthcare provider.
How much to use
Your doctor will determine the correct dose of STELARA for you and how often you should receive it. Make sure to discuss with your doctor when you will receive injections and to come in for all your scheduled follow-up appointments.
For children and adolescents aged 6 years or older with psoriasis:
The doctor will work out the right dose for you, including the amount (volume) of Stelara to be injected to give the right dose. The right dose for you will depend on your body weight at the time each dose is given. If you weigh less than 60 kg, the recommended dose is 0.75 mg of Stelara per kg body weight.
If you weigh 60 kg to 100 kg, the recommended dose is 45 mg Stelara.
If you weigh more than 100 kg, the recommended dose is 90 mg Stelara.
After the starting dose, you will have the next dose 4 weeks later, and then every 12 weeks.
Injecting STELARA under the skin yourself
Your first STELARA injection may be administered by your healthcare provider. In children and adolescents 6 years and older, it is recommended that all doses of STELARA be administered by a healthcare provider. However, your healthcare provider may decide that it is right for you or your caregiver to learn how to inject STELARA under the skin (subcutaneously) yourself. If you would like to self-inject STELARA under your skin, you or your caregiver must be trained by a healthcare professional to prepare an injection and give it to yourself. If you have not been trained, please contact your healthcare provider to schedule a training session. Call your healthcare provider if you have any questions about giving yourself an injection.
STELARA is given alone and not mixed with other liquids for injection.
STELARA should not be used:
after the expiry date on the label;
if the seal is broken;
if the liquid is discoloured, cloudy or you can see other particulate matter floating in it;
if you know, or think that it may have been exposed to extreme temperatures (such as being accidentally frozen or heated).
Do not shake STELARA vials at any time.
Prolonged vigorous shaking may damage the product. If STELARA has been shaken vigorously, don’t use it. STELARA is not to be mixed with other liquids for injection.
How to inject STELARA from a vial
1.Check Vials and Assemble materials:
Take the vial(s) out of the refrigerator. Make sure that it is the right dose. If your dose is 45 mg you will receive one 45 mg vial. If your dose is 90mg, you will receive two 45 mg vials. If you receive two 45 mg vials for a 90 mg dose, you will need to give yourself two injections one right after the other. Check with your healthcare provider.
Children weighing less than 60 kg require a dose lower than 45 mg. Make sure you know the proper amount (volume) and type of syringe needed for dosing. If you don’t know the amount or type of syringe needed, contact your healthcare provider for further instructions.
Check Expiration Date
Open the box and remove the vial. Check the expiration date on the vial and the label of the box. If the expiration date has passed, don’t use it.
Check Solution in Vial
Make sure the vial is not damaged. Look at the solution or liquid in the vial to make sure that it is not cloudy and not frozen.
Assemble Additional Supplies
Assemble the additional supplies you will need for your injection. These include a syringe with a 27 gauge, 1/2 inch needle, an alcohol swab, a cotton ball or gauze, and a sharps container for syringe disposal.
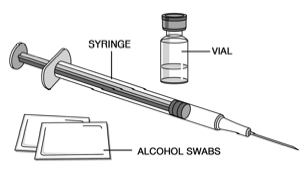
2.Choose the Injection Site:
Good sites are the top of the thigh and around the tummy (abdomen) but about 2 inches away from the belly button (navel). Avoid, if possible, skin involved with psoriasis. If your caregiver is giving you the injection, they may use the upper arms or buttocks as well.
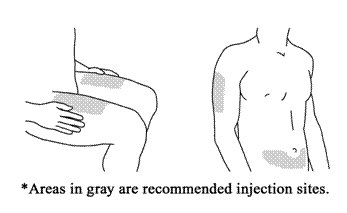
Prepare the Injection Site. Thoroughly wash your hands with soap and warm water. Wipe the injection site with an alcohol swab. DO NOT touch this area again before giving the injection.
3.Preparing the dose:
Remove the cap from the top of the vial but do not remove the stopper.
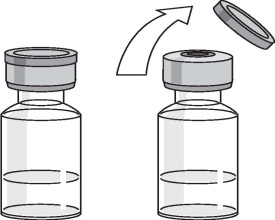
Clean the stopper with an antiseptic swab.
Remove the needle cover from the syringe. Do not touch the needle or allow the needle to touch anything.
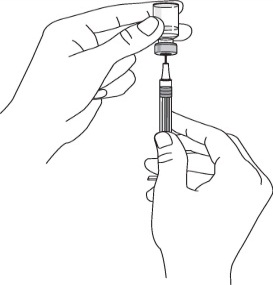
Put the vial on a flat surface and push the syringe needle through the rubber stopper.
Turn the vial and the syringe upside down. For adults and paediatric patients (children and adolescents) 6 years of age and older, who weigh 60 kg or more, pull on the syringe plunger to fill the syringe with the amount of liquid prescribed by your healthcare provider (0.5 mL or 1.0 mL).
For children and adolescents 6 years of age or older who weigh less than 60 kg, the amount of liquid prescribed by your healthcare provider may be less than 0.5 mL. Your healthcare provider will recommend how much liquid is needed.
It is important that the needle is always in the liquid in order to prevent air bubbles from forming in the syringe.
Remove the needle from the vial. Hold the syringe with the needle pointing up to see if it has any air bubbles inside.
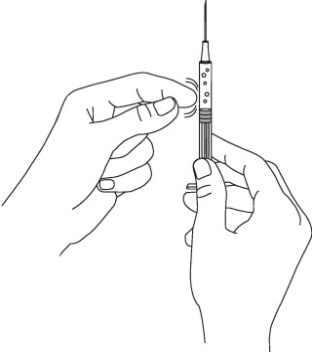
If there are air bubbles tap the side gently until the air bubbles go to the top of the syringe and press the plunger until all of the air (but none of the liquid) has been removed. Do not lay the syringe down or allow the needle to touch anything.
4.Injecting the Medication:
Gently pinch the cleaned skin between your thumb and index finger. Don’t squeeze it.
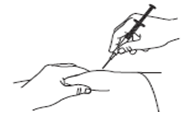
Push the syringe needle into the pinched skin.
Push the plunger with your thumb as far as it will go to inject all of the liquid. Push it slowly and evenly, keeping the skin gently pinched.
When the plunger is pushed as far as it will go, take out the needle and let go of the skin.
Press an antiseptic swab over the injection site for a few seconds after the injection.
Dispose of the Empty Syringe
Immediately dispose of the empty syringe into the sharps container. For your safety and health and for the safety of others, needles and syringes must NEVER be re-used. Dispose of sharps container according to your local regulations. See “After using STELARA – Disposal”.
Use a Cotton Ball or Gauze
There may be a small amount of blood or liquid at the injection site, which is normal. You can press a cotton ball or gauze over the injection site and hold for 10 seconds. Do not rub the injection site. You may cover the injection site with a small adhesive bandage, if necessary.
If your dose amount is 90 mg, and you receive two 45 mg vials you may need to give a second injection right after the first. Use a new needle and syringe. Choose a different site for the second injection.
If you do not understand the instructions provided with this medicine, ask your doctor or pharmacist for help.
If you forget to use it
Make the next injection as soon as you remember, and then continue to use it as you would normally.
Do not administer a double dose to make up for the dose you missed.
If you have missed more than one dose, or are not sure what to do, check with your doctor or pharmacist.
If you have trouble remembering when to use your medicine, ask your pharmacist for some hints.
If you have used too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre for advice or go to Accident and Emergency at your nearest hospital.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Poisons Information Centre telephone numbers:
Australia: 13 11 26
New Zealand: 0800 POISON or 0800 764 766
Keep these telephone numbers handy.
While you are using STELARA
Things you must do
Always follow your doctor’s instructions carefully.
Tell your doctor about your medical conditions before each treatment (see Before you use STELARA).
Tell your doctor if you become pregnant while using STELARA.
If you are about to start taking a new medicine, tell your doctor and pharmacist that you are using STELARA.
Things you must not do
You should not receive a live vaccine while taking STELARA.
Do not use STELARA to treat any other complaint unless your doctor says so.
Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
Side Effects
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some side effects. Do not be alarmed by this list of possible side effects. You may not experience any of them.
In general, the side effects of STELARA in children and adolescents 6 years and older are similar to those in adults. Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you experience any of the following common side effects:
Upper respiratory infections such as sinus infection and colds
Dizziness, headache
Sore throat
Diarrhoea
Nausea, vomiting
Pruritis (itching)
Back or muscle pain
Joint pain
Injection site pain or redness
Fatigue (tiredness)
Sinus infection
Other uncommonly reported side effects include:
Painful skin rash (Herpes Zoster infection)
Cellulitis
Dental infections
Viral upper respiratory tract infection
Vaginal yeast (fungal) infection
Depression
Blocked or stuffy nose
Acne
Injection site reactions (including bleeding, bruising, hardness, swelling and itch)
Feeling weak
A change in psoriasis with redness and new tiny, yellow or white skin blisters, sometimes accompanied by fever (pustular psoriasis)
Worsening reddening or shedding/peeling of the skin
Lower respiratory tract infections such as bronchitis or pneumonia
Tell your doctor immediately or go to hospital as you need urgent medical attention:
A serious allergic reaction. Signs of a serious allergic reaction may include a skin rash, a swollen face, lips, mouth or throat, or wheezing, dizziness, trouble swallowing or breathing.
In rare cases, symptoms such as cough, shortness of breath, and fever may also be a sign of an allergic lung reaction to STELARA.
STELARA is a medicine that may decrease the activity of your immune system. It can increase your chances of getting serious side effects. Tell your doctor immediately if you notice any of the following, as you may need urgent medical care:
Serious Infections. STELARA may lower your ability to fight infections. Some infections could become serious and lead to hospitalization. If you have an infection, tell your healthcare provider before you start using STELARA. If you get an infection, have any sign of an infection such as fever, feel very tired, cough, flu-like symptoms, or have any open cuts or sores, tell your healthcare provider right away.
Cancer. Many drugs such as STELARA that may decrease the activity of the immune system may increase the risk of cancer. Tell your doctor if you have ever had any type of cancer.
Other side effects not listed above may also occur in some people. Tell your doctor if you notice any other side effects, particularly if they bother you or do not go away. In general, the side effects of STELARA in children and adolescents 6 years and older are similar to those in adults. Ask your doctor or pharmacist for more information.
After using STELARA
Storage
Store STELARA between 2°C and 8°C in the refrigerator. Do not freeze. Keep the product in the original carton to protect from light until the time of use. Do not shake.
Keep your medicines out of reach of children.
Do not store STELARA, or any other medicine, in the bathroom or near a sink. Do not leave medicines in the car or on windowsills. Heat and dampness can destroy some medicines.
Disposal
After injection, used syringes should be placed in a puncture-resistant container, like a sharps container. Dispose of your sharps container according to your state or local regulations. Empty vials, antiseptic wipes, and other supplies can be placed in regular rubbish.
If your doctor tells you to stop using STELARA, or your medicine has passed its expiry date, ask your pharmacist what to do with any medicine that may be left over.
Product Description
What it looks like
STELARA injection for subcutaneous use is a colourless to light yellow solution and may contain a few small translucent or white particles of protein. This appearance is not unusual for solutions containing protein.
STELARA solution for intravenous use is a clear, colourless to light yellow product.
STELARA is available in glass vials containing 45mg ustekinumab in 0.5mL (for subcutaneous use) or 130 mg in 26 mL (for intravenous use). Each box contains 1vial.
Australian Registration numbers:
STELARA 45mg AUST R 149549
STELARA 130mg AUST R 282906
Ingredients
STELARA 45 mg solution for injection for subcutaneous administration
Each vial contains 45 mg of ustekinumab.
Inactive ingredients: histidine/histidine hydrochloride monohydrate, sucrose, polysorbate 80, and water for injections.
No preservatives are present.
STELARA 130 mg solution for intravenous infusion
Each vial contains 130 mg of ustekinumab.
Inactive ingredients: L-histidine, L-histidine hydrochloride monohydrate, sucrose, polysorbate 80, L-methionine, disodium edetate, and water for injections.
No preservatives are present.
Sponsor
JANSSEN-CILAG Pty Ltd
1-5 Khartoum Road
Macquarie Park NSW 2113 Australia
Telephone: 1800 226 334
NZ Office: Auckland New Zealand
Telephone: 0800 800 806
This leaflet was prepared in November 2020.
Nurse and educational support
The Patient Support Program is available to patients prescribed STELARA and offers:
home visits from a qualified STELARA nurse to provide self-injection education
injection SMS, phone or email reminders
educational resources
Call 1800 666 845
Source: Read Full Article
