Skip to:
- Fischer projections
- Haworth projections
- Choices between the Fischer and Haworth projections
In the field of stereochemistry, the structure of a compound and the three-dimensional arrangement of its atoms needs to be known to understand the behavioural and chemical properties of the compound. Whilst molecular entities are by their nature three-dimensional, they are commonly depicted on two-dimensional media. Therefore, a certain degree of distortion is needed to accurately convey the three-dimensional structure.
In organic chemistry, there are conventions for illustrating bonds within a molecule. A wedge-dash notation is used to denote orientation of the structure. Dotted lines are used to represent an atomic bond which is further away from the viewer, a wedge for the atoms nearer, and a solid line to show a bond in plane with the molecule.
Fischer and Haworth projections are two types of illustration which are used to represent the 3D arrangement of atoms in carbohydrates. They are also used to compare different carbohydrates.
Fischer projections
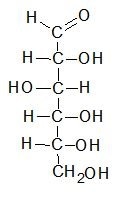
The Fischer projection was devised by German chemist Emil Fischer in 1891, who was the winner of the Nobel Prize in Chemistry in 1902. In a Fischer projection the carbohydrate is shown in its open chain form, rather than a cyclical one. Carbon atoms in the main chain of the carbohydrate molecule are connected vertically, whilst hydrogen atoms and hydroxyl groups are bonded horizontally. The horizontal lines illustrate the bonds which come out of the page, whereas the vertical lines show bonds that are in the page. Carbon atoms may or may not be shown in a Fischer projection.
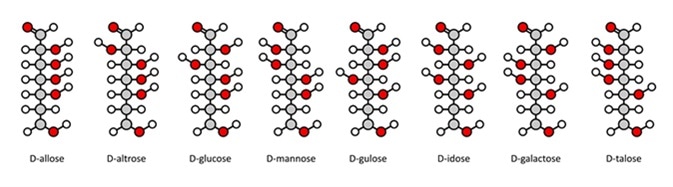
Fischer projections have one main advantage: it is easy to visually identify the stereochemical properties of a carbohydrate and compare the difference between two carbohydrates quickly and easily. For example, it is simple to tell the difference between two enantiomers (molecules that are a mirror image of each other.)
A Fischer projection can be rotated 180 degrees without affecting the molecule’s stereoisomerism. If it were to be rotated 90 degrees then a different enantiomer would be illustrated, so therefore this is impossible with a Fischer projection. Small changes can affect a molecule’s characteristics: therefore, care must be taken when using Fischer projections to illustrate a carbohydrate.
As Fischer projections can have a degree of ambiguity when confused with other types of drawing, their use to represent non-carbohydrates is discouraged. They are mainly used to illustrate monosaccharides. They can be used to represent other organic molecules including amino acids, but this is discouraged by the 2006 IUPAC recommendations. IUPAC rules also determine that the hydrogen atoms should be explicitly drawn especially the hydrogen atoms of the end group of carbohydrates. Fischer projections are different to skeletal formulae.
Below is a Fischer projection of D-glucose in its open chain form.
Haworth projections
A Haworth projection differs from a Fischer projection in that it is used to represent the carbohydrate in its cyclical form. This is especially useful for sugars which have a ring structure. It was devised by the English chemist Sir Norman Haworth who expanded on the work of Fischer, characterizing many more carbohydrates. He developed the illustration technique after World War 1, and received the 1937 Nobel Prize for Chemistry for his work in investigating carbohydrates and Vitamin C.
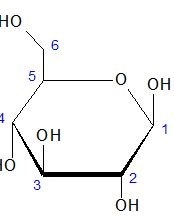
In a Haworth projection, which is now the standard in organic chemistry for stereochemical carbohydrate illustrations, thicker bonds between carbon atoms represent those closest to the viewer, and the hydrogen/hydroxyl bonds below the plane of the carbon atoms represent those on the right in a Fischer projection. However, this rule does not apply to the groups on the two ring carbons bonded to the endocyclic oxygen atom.
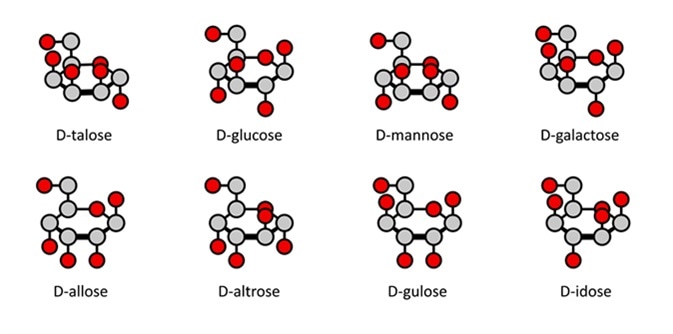
Compared to a Fischer projection, where carbon atoms may or may not be illustrated, in a Haworth projection they are implied. Carbon 1 is also referred to as the anomeric carbon. Hydrogen atoms which are bonded to a carbon atom are also implied, so are not shown. Hydroxyl groups and other atoms which may be bonded to the carbon ring are shown.
One drawback to Haworth projections is that they are not entirely accurate in representing the spatial positioning of all the atoms. A “chair” conformation can be used to more accurately depict the spatial positioning of the atoms; however, this conformation can make determination of the carbohydrate’s basic stereochemistry more difficult.
Below is the Haworth projection for the cyclic form of D-glucose.
Choices between the Fischer and Haworth projections
As can be seen, both Fischer and Haworth projections are two standard ways of illustrating the stereochemistry of a carbohydrate. Whilst the Haworth projection is more commonly used these days, and a chair conformation can help to depict the atoms position in space, the Fischer projection certainly still has its uses in organic chemistry. The choice between the two conventions is down to the individual chemist.
Sources
- Graphical representation of stereochemical configuration (IUPAC Recommendations 2006), http://publications.iupac.org/pac/2006/pdf/7810×1897.pdf
- Moreno, L.F. (2012), Understanding Fischer Projection and Angular Line Representation Conversion, J. Chem. Educ. Vol. 89 Issue 1 pgs.175-176 , https://doi.org/10.1021/ed101011c
Further Reading
- All Stereochemistry Content
Last Updated: Oct 22, 2019

Written by
Reginald Davey
Reg Davey is a freelance copywriter and editor based in Nottingham in the United Kingdom. Writing for News Medical represents the coming together of various interests and fields he has been interested and involved in over the years, including Microbiology, Biomedical Sciences, and Environmental Science.
Source: Read Full Article