NOTICE: This Consumer Medicine Information (CMI) is intended for persons living in Australia.
BEOVU®
This medicine is subject to additional monitoring in Australia. This will allow quickidentification of new safety information. You can help by reporting any side effectsyou may get. You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems.
solution for injection
brolucizumab (rbe)
Consumer Medicine Information
What is in this leaflet
This leaflet answers some common questions about Beovu.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine. You can also download the most up to date leaflet from www.novartis.com.au
Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Beovu against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What Beovu is used for
Beovu is a prescription medicine that is injected into the eye by your doctor to treat an eye disorder called neovascular (wet) age-related macular degeneration (AMD). Beovu contains the active substance brolucizumab (bro-loo-siz-u-mab), which belongs to a group of medicines called anti- neovascularization agents (“anti- VEGF”, see below).
Beovu is used to treat wet AMD, which occurs when abnormal blood vessels form and grow underneath the macula. The macula is located at the back of the eye, and it is responsible for clear vision. These abnormal blood vessels may be weak and leak fluid or blood in the eye, which can interfere with the macula’s function, resulting in decreased vision.
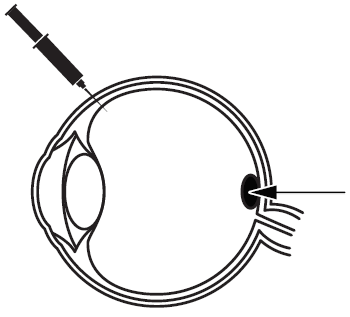
Abnormal blood vessels that leak fluid or blood into the macula
The cause for the abnormal growth of blood vessels in the eye is a substance called vascular endothelial growth factor A (VEGF-A), which the body produces in large amounts. By attaching to this substance, Beovu (anti-VEGF-agent) lessens the growth of new blood vessels, which in turn reduces the leakage of fluid or blood in the eye.
If you have any questions about Beovu, how it works or why this medicine has been prescribed for you, ask your doctor.
Beovu may slow down disease progression and thereby maintain, or even improve your vision.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
This medicine is not addictive.
It is available only with a doctor’s prescription.
There is not enough information to recommend the use of this medicine for children or adolescents.
Before you have Beovu
When you must not take it
You must not be given Beovu:
If you are allergic (hypersensitive) to brolucizumab or any of the other ingredients in Beovu.
If you have an active or suspected infection in or around the eye.
If you experience pain or redness in your eye.
If any of these apply to you, tell your doctor. You should not be given Beovu.
You must not be given this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should be given this medicine, talk to your doctor.
Before you are given it
It is important to tell your doctor if any of the following apply to you before Beovu is injected:
If you have glaucoma.
If you have a history of seeing flashes of light or floaters (dark floating spots) and if you have a sudden increase of size and number of floaters.
If surgery was performed or is planned on your eye within the previous or next four weeks.
If you have a prior history of eye conditions or eye treatments.
If you ever had a stroke or heart attack. Signs of a stroke may include weakness or numbness of limbs or face, difficulty speaking or swallowing. Signs of a heart attack may include chest pain, which may spread to the neck and shoulders.
Your doctor will evaluate this information to decide if Beovu is right for you.
Tell your doctor if you are pregnant, think you are pregnant or plan to become pregnant.
Your doctor can discuss with you the risks and benefits involved.
Tell your doctor if you have the potential to become pregnant.
It is recommended that you use effective contraception during Beovu treatment and for at least one month after the last injection of Beovu.
Tell your doctor if you are breastfeeding or planning to breast-feed.
Beovu is not recommended during breast feeding and for at least one month after the last injection when stopping treatment with Beovu. It is not known whether Beovu passes into breast milk.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How Beovu is given
Beovu is given by your ophthalmologist (eye doctor) as an injection into your eye under a local anaesthetic.
Follow the directions given to you by your doctor carefully.
They may differ from the information contained in this leaflet.
If you do not understand the instructions, ask your doctor for help.
It is important to see your doctor regularly when on treatment with Beovu.
How much is given
The recommended dose is 6 mg (0.05 mL) of brolucizumab.
You will be treated with one injection per month for the first three months.
After that, you may get one injection every eight (2 months) to twelve weeks (3 months). The interval between two doses should be no shorter than 4 weeks (1 month). Your doctor will determine your treatment interval based on the condition of your eye.
Once you begin receiving Beovu, it is important to follow the treatment schedule recommended by your doctor. This could help you receive the full potential benefit of Beovu.
How it is given
Beovu is given as an injection into your eye (intravitreal injection).
Before the injection, your doctor will use a disinfectant eyewash to clean your eye carefully, to prevent infection. Your doctor will also give you an eye drop (local anesthetic) to numb the eye to reduce or prevent any pain you might have with the injection.
How long does Beovu treatment continue
Wet AMD is a chronic disease, therefore this is a long-term treatment, possibly continuing for months or years. Your doctor will check that the treatment is having the desired effect during your regularly scheduled visits. Your doctor may also check your eyes during a visit without an injection.
If you have questions about how long you will receive Beovu, talk to your doctor.
If a scheduled Beovu injection is missed
Missing an injection may reverse the visual improvement you may have experienced.
If you miss an appointment for Beovu treatment, contact your doctor as soon as possible.
Your doctor will decide when you should be given your next dose.
Before stopping Beovu treatment
Speak with your doctor before stopping treatment.
Stopping treatment may increase your risk of vision loss and reverse the visual improvement you may have experienced.
If you have any further questions on the use of this medicine, ask your doctor.
If you are given too much (overdose)
If you are given more Beovu than you need, your doctor will check the pressure in your eye and may need to treat it if it is increased.
While you are being given Beovu
Things you must do
If you experience any problems during the treatment, tell your doctor.
Tell your doctor immediately if you get any of these symptoms after Beovu is injected:
If you develop redness of the eye or worsening eye redness, eye pain, increased discomfort, any change in vision (such as sudden vision loss, blurred or decreased vision), an increased number of small particles also commonly referred to as floaters, seeing spots or cobwebs in your vision, increased sensitivity to light, nausea or vomiting. All of these could be symptoms of a serious eye condition.
A serious eye infection or eye disorder can sometimes develop after an injection into the eye.
Tell your doctor if you notice any bruising or unexplained bleeding.
There is a theoretical increased risk of bleeding with this group of medicines (anti-VEGF).
If you become pregnant while being treated with this medicine, tell your doctor immediately.
Things you must not do
Do not drive or operate machinery if your vision is poor, either because of your disease or because of the treatment.
After your injection with Beovu, you may experience some temporary vision problems (example – blurry vision). If you are affected, do not drive, operate machinery or do anything else that could be dangerous until your vision is normal.
Side effects
As with all medicines, patients treated with Beovu may experience side effects, although not everybody gets them. The side effects associated with the administration of Beovu are either due to the medicine itself or the injection procedure and mostly affect the eye.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are being treated with Beovu.
Some side effects could be serious
Tell your doctor or pharmacist if you notice any of the following and they worry you:
eye irritation, clouding of the lens, a feeling of having something in the eye, dry eye, abnormal sensation in the eye
eye discomfort, pain or irritation at the site of the injection, increased tear production, redness or itching of the eye, small particles or spots in your vision (floaters)
allergic reactions (rash, itching, redness of the skin).
The above list includes side effects of your medicine which are usually mild and short-lived.
Tell your doctor as soon as possible if you notice any of the following:
bloodshot eye, bleeding in the eye, inflammation or infection of the eyelid margins, visual disturbance, blurred or decreased sharpness of vision, blindness (temporary or otherwise)
discharge of the eye with itching, redness and swelling (conjunctivitis)
small marks on the surface of the eye, swelling of a section of the eye (cornea, uvea), swelling or irritation of the eyelid, eyelid pain, sac of pus on the eye.
The above list includes serious side effects which may require medical attention. Serious side effects are rare.
Tell your doctor immediately if you experience any of the following:
signs of inflammation or infection of the eye such as redness or worsening redness of the eye, eye pain, sensitivity to light, any vision changes, including sudden vision loss.
seeing flashes of light with floaters (seeing spots or cobwebs), progressing to blurred vision or loss of sight.
signs of a stroke, such as weakness or numbness of limbs or face, difficulty speaking or swallowing.
signs of a heart attack may include chest pain, which may spread to the neck and shoulders.
The above are serious side effects which might need immediate medical attention.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people. Some of these side effects (e.g. an increase in the pressure inside your eye) can only be found when your doctor does tests to check your progress.
How to store Beovu
The information on how to store Beovu is meant for your doctor, who will be storing, handling, and injecting Beovu.
Storage
If you have to store Beovu:
Pre-filled syringe
Keep it in a refrigerator (2°C to 8°C). Do not freeze it.
Keep the pre-filled syringe in the sealed blister and in the outer carton in order to protect from light.
Prior to use, the unopened blister may be kept at room temperature (25°C) for up to 24 hours.
Vial
Keep it in a refrigerator (2°C to 8°C). Do not freeze it.
Keep the vial in the outer carton in order to protect from light.
Prior to use, the unopened vial may be kept at room temperature (25°C) for up to 24 hours.
Do not store Beovu or any other medicine in the bathroom or near a sink.
Do not leave it in the car or on a window sill.
Keep the medicine where young children cannot reach it.
Product description
What it looks like
Pre-filled syringe
Beovu is a solution for injection supplied in a glass pre-filled syringe. The pre-filled syringe contains 0.165 mL of a clear to slightly opalescent, colourless to slightly brownish-yellow aqueous solution.
Beovu is supplied as packs containing one single use sterile pre-filled syringe in a sealed tray.
Vial
Beovu is a solution for injection supplied in a glass vial. The vial contains 0.230 mL of a clear to slightly opalescent, colourless to slightly brownish-yellow aqueous solution.
Beovu is supplied as packs containing one single use glass vial and one filter needle for withdrawal of the vial contents.
Not all presentations may be marketed.
Ingredients
Beovu pre-filled syringe contains 19.8 mg brolucizumab as the active ingredient.
Beovu vial contains 27.6 mg brolucizumab as the active ingredient.
The pre-filled syringe and vial also contain:
sodium citrate
sucrose
polysorbate 80
water for injections
Allergens:
This medicine contains sorbates.
Sponsor
Beovu is supplied in Australia by:
Novartis Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1 800 671 203
®= Registered Trademark
This leaflet was prepared in
June 2020.
Australian Registration Numbers:
AUST R 313681– 19.8 mg/0.165 mL solution for injection in pre-filled syringe
AUST R 313680– 27.6 mg/0.230 mL solution for injection in vial
(beo250620c based on PI beo250620i)
Source: Read Full Article
