Nearly 150,000 cancer-related deaths can be attributed annually to Epstein-Barr (EBV) virus, in part because of the lack of effective treatment options.
Now, a research team has created the first comprehensive map of interactions between the genes of the virus and host cells in EBV-associated cancers, knowledge that could lead to new treatments. The research is a collaboration of scientists from Purdue University, the National Institutes of Health and Harvard University.
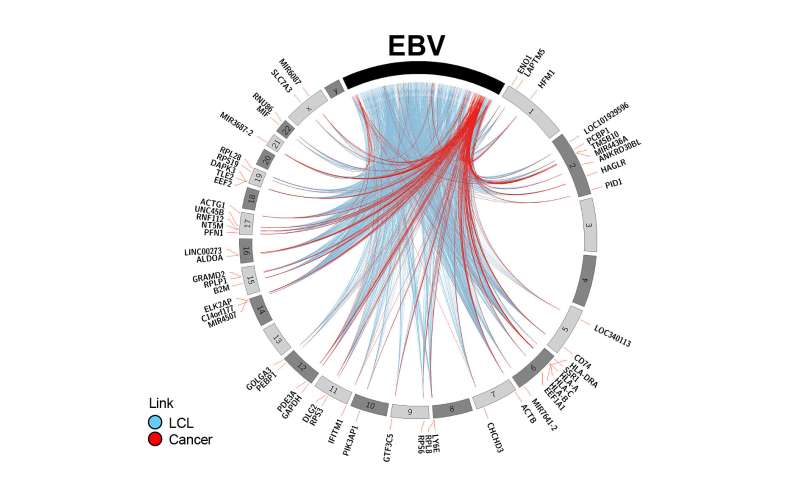
The research team has created an “interactome map,” which shows how the expression, mutation and integration of viral genes affect the host genes and vice versa.
“In this collaboration between the Purdue University Center for Cancer Research, the NIH’s National Institute of Diabetes and Digestive and Kidney Diseases, and Harvard University, we developed the first comprehensive map of interactions between Epstein-Barr virus and host cells in EBV-associated cancers,” said Majid Kazemian, an assistant professor in Purdue’s departments of Biochemistry and Computer science. “The molecular mechanisms governing Epstein-Barr virus’s carcinogenesis remain elusive, and the functional interactions between virus and host cells are incompletely defined. Here, we present a comprehensive map showing the interactions of host cell and pathogen genes in EBV-associated cancers.”
Research findings were published online Sept. 3 in the journal Cancer Research. The paper’s lead author was Purdue biochemistry graduate student Srishti Chakravorty.
The Epstein-Barr virus is ubiquitous, infecting approximately 90 percent of the world’s adult population. In its latent stage, the virus causes a wide range of cancers. Over the five decades since the discovery of this multi-disease associated oncovirus, various aspects of its life cycle and host immune response in individual tumor types or cell lines have been studied.
“However, generalized host-virus interactions in a large cohorts of cancer patients have not been systematically delineated,” Kazemian said.
The researchers systematically analyzed genetic sequencing from more than 1,000 patients with 15 different cancer types.
“Overall, these findings uncover novel points of interaction between a common oncovirus and the human genome and identify new regulatory nodes and druggable targets for individualized EBV and cancer-specific therapies,” Kazemian said.
The research is ongoing.
Source: Read Full Article
